Acute toxicity test of monosodium glutamate in Channa punctatus and its ecotoxicological implications
DOI:
https://doi.org/10.63697/jeshs.2025.10048Keywords:
Monosodium glutamate (MSG), Food additive, LC50, Channa punctatus, Acute toxicityAbstract
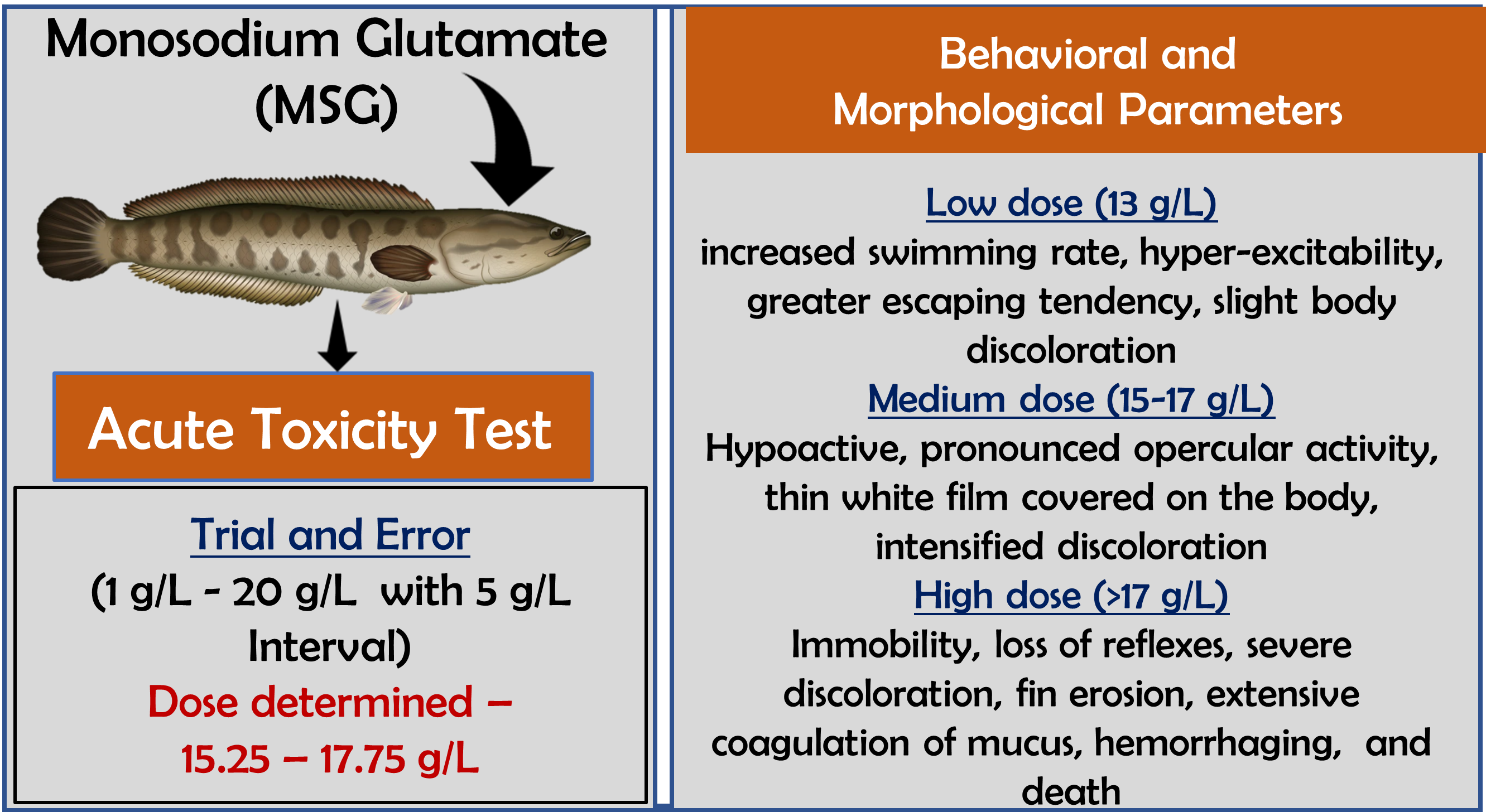
Monosodium glutamate (MSG), the umami substance commonly used in the food industry, is known for its ability to enhance savory flavors. Although it is generally recognized as safe by Food Safety Regulatory Agencies, several studies have questioned its long-term safety. Despite its widespread use, the potential toxicity of MSG therefore remains under-researched. This study aimed to evaluate the acute toxicity of MSG in terms of its median lethal concentrations (LC50) as well as the potential to induce morphological and behavioral changes in the freshwater fish Channa punctatus upon short-term exposure. Static bioassay tests were conducted by exposing the fish to different concentrations of MSG and the mortality of the fish was recorded at 24, 48, 72, and 96 hours of exposure. The physicochemical parameters of the aquarium water, such as pH, temperature, dissolved oxygen, total hardness, and total alkalinity, were maintained within their optimal ranges. The LC50 value was determined using probit analysis following standard guidelines to ensure accuracy and reproducibility. The results indicate that MSG exhibited acute toxicity to C. punctatus within the tested range of concentrations. Furthermore, a dose-dependent increase in mortality as well as marked behavioral and morphological changes were observed. The severity of symptoms increases at higher MSG concentrations, indicating induction of physiological stress in exposed fish. This is the first study to assess the acute toxicity of MSG on the fish C. punctatus. These findings provide baseline information that will aid further toxicological research and monitoring of the long-term effects of MSG on aquatic organisms.
Downloads
References
Abu Zeid, E.H., Khalifa, B.A., Said, E.N., Arisha, A.H., Reda, R.M., 2021. Neurobehavioral and immune-toxic impairments induced by organic methyl mercury dietary exposure in Nile tilapia Oreochromis niloticus. Aquatic toxicology, 230, 105702. https://doi.org/10.1016/j.aquatox.2020.105702
Adeyemo, O.A., Farinmade, A.E., 2013. Genotoxic and cytotoxic effects of food flavour enhancer monosodium glutamate (MSG) using Allium cepa assay. African Journal of Biotechnology, 12, 1459–1466.
Ajibade, A.J., Fakunle, P.B., Adetunji, M.O., 2015. Some effects of monosodium glutamate administration on the histo-architecture of the spleen and pancreas of adult Wistar rats. Journal of Pharmacy and Biological Science, 3, 39–50.
Al-Mosaibih, M.A., 2013. Effects of monosodium glutamate and acrylamide on the liver tissue of adult Wistar rats. Life Science Journal, 10, 35–42.
Alalwani, A.D., 2014. Monosodium glutamate induced testicular lesions in rats (histological study). Middle East Fertility Society Journal, 19, 274–280. https://doi.org/10.1016/j.mefs.2013.09.003
American Public Health Association (APHA), American Water Works Association, Water Environment Federation. Lipps WC, Braun-Howland EB, Baxter TE, eds. Standard Methods for the Examination of Water and Wastewater. 24th ed. Washington DC: APHA Press; 2023.
Burden, N., Benstead, R., Benyon, K., Clook, M., Green, C., Handley, J., Harper, N., Maynard, S.K., Mead, C., Pearson, A., Ryder, K., Sheahan, D., van Egmond, R., Wheeler, J.R., Hutchinson, T.H., 2020. Key opportunities to replace, reduce and refine regulatory fish acute toxicity tests. Environmental Toxicology and Chemistry, 10, 2076–2089. https://doi.org/10.1002/etc.4824
Chakraborty, S.P., 2019. Patho-physiological and toxicological aspects of monosodium glutamate. Toxicological Mechanisms and Methods, 29, 389–396. https://doi.org/10.1080/15376516.2018.1528649
Chaohua, L., 1994. Studies on heavy metals in commercial fishes from the northern part of South China Sea. Journal of Fishery Sciences of China, 1, 68–77.
Dean, J.G., Bosqui, F.L., Lanouette, K.H., 1972. Removing heavy metals from wastewater. Environmental Science & Technology, 6, 518–522. https://doi.org/10.1021/es60065a006
Essawy, A.E., Jimmiey, E.M., Abdel-Wahab, W.M., Ali, R.G., Eweda, S.M., Abdou, H.M., 2025. Protective efficacy of omega-3 fatty acids on oxidative stress, inflammation, neurotransmitter perturbations and apoptosis induced by monosodium glutamate in the brain of male rats. Metabolic Brain Disease, 40, 114. https://doi.org/10.1007/s11011-025-01539-4
FAO & WHO, 2008. Dietary exposure assessment of chemicals in food. Report of Joint FAO/WHO Consultation, Annapolis, USA, 6, 1–130. https://www.who.int/docs/default-source/chemical-safety/ehc240-chapter6-edited(4-1).pdf?sfvrsn=96810319_0
Farombi, E.O., Onyema, O.O., 2006. Monosodium glutamate–induced oxidative damage and genotoxicity in rats: modulatory role of vitamin C, vitamin E and quercetin. Human & Experimental Toxicology, 25, 251–259. https://doi.org/10.1191/0960327106ht621oa
Flores-Lopes, F., Thomaz, A.T., 2011. Histopathologic alterations in fish gills as tools for environmental monitoring. Brazilian Journal of Biology, 71, 179–188. https://doi.org/10.1590/S1519-69842011000100026
Formicki, G., Goc, Z., Bojarski, B., Witeska, M., 2025. Oxidative stress and neurotoxicity biomarkers in fish toxicology. Antioxidants, 14, 936. https://doi.org/10.3390/antiox14080939
Gabriel, U.U., Uedeme-Naa, B., Akinrotimi, O.A., 2011. Pollutant-induced altered behaviours in fish: A review. Journal of Technology and Education in Nigeria, 16, 9–26.
Hellou, J., 2011. Behavioural ecotoxicology: An early-warning tool for assessing environmental quality. Environmental Science and Pollution Research, 18, 1–11. https://doi.org/10.1007/s11356-010-0367-2
Hoyle, I., Oidtmann, B., Ellis, T., Turnbull, J., North, B., Nikolaidis, J., Knowles, T.G., 2007. A validated macroscopic key for assessing fin damage in farmed rainbow trout (Oncorhynchus mykiss). Aquaculture, 270, 142–148. https://doi.org/10.1016/j.aquaculture.2007.03.037
Hrovat, M., Segner, H., Jeram, S., 2009. Variability of in vivo fish acute toxicity data. Regulatory Toxicology and Pharmacology, 54, 294–300. https://doi.org/10.1016/j.yrtph.2009.05.013
Ibrahim, M.M., Mahmoud, M.A., 2024. Pathological studies on skeletal muscle atrophy in fish products from El-Jubail Province, Saudi Arabia. Scientific Reports, 14, 30594. https://doi.org/10.1038/s41598-024-76880-2
Islam, M.A., Amin, S.M.N., Brown, C.L., Juraimi, A.S., Uddin, M.K., Arshad, A., 2021. Determination of median lethal concentration (lc50) for endosulfan, heptachlor and dieldrin pesticides to african catfish, Clarias gariepinus and their impact on its behavioral patterns and histopathological responses. Toxics, 9, 340. https://doi.org/10.3390/toxics9120340
Jha, D.K., Sayrav, K., Mishra, G.P., Mishra, B.B., Kumari, A., Kumar, A., Khan, P.K., 2019. Risk assessment of low arsenic exposure using biomarkers of oxidative and genotoxic stress in a piscine model. Ecotoxicology, 28, 669–679. https://doi.org/10.1007/s10646-019-02060-y
Jinap, S., Hajeb, P., 2010. Glutamate. Its applications in food and contribution to health. Appetite, 55, 1–10. https://doi.org/10.1016/j.appet.2010.05.002
Kurnianingsih, N., Utami, J.P., Nurdiana, Lyrawati, D., 2016. Monosodium glutamate exposure during early development increases apoptosis and stereotypic behaviour in zebrafish larvae. Indonesian Journal of Pharmacy, 27, 128–138. https://doi.org/10.14499/indonesianjpharm27iss3pp128
Lau, A., Tymianski, M., 2010. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Archiv – European journal of physiology, 460, 525–542. https://doi.org/10.1007/s00424-010-0809-1
Lewis, J.A., Finney, D., 1972. Probit Analysis (3rd ed.). Applied Statistics, 21. https://doi.org/10.2307/2346498
Li, F-Y., Yang, W-H., Chang, C-I., Lee, S.J., Hung, C.C., Chen, Y.J., Jinn, T.R., Tzen, J.T.C., 2013. Concurrent accumulation of myricetin and gallic acid putatively responsible for the umami taste of specialized old Oolong tea. Journal of Food and Nutrition Research, 1, 164–173. https://doi.org/10.12691/jfnr-1-6-8
Liu, R., Zhou, Q., Zhang, L., Guo, H., 2007. Toxic effects of MSG wastewater on seed germination and root elongation. Frontiers of Environmental Science & Engineering, 1, 114–119. https://doi.org/10.1007/s11783-007-0021-5
Loganathan, K., Tennyson, S., Arivoli, S., 2024. Triazophos toxicity-induced histological abnormalities in Heteropneustes fossilis. Journal of Basic and Applied Zoology, 85, 19. https://doi.org/10.1186/s41936-024-00373-x
Madelaine, R., Ngo, K.J., Skariah, G., Mourrain, P., 2020. Genetic control of pigmentation pathways in zebrafish. PLoS Genetics, 16. https://doi.org/10.1371/journal.pgen.1009244
Magnoni, L.J., Crespo, D., Ibarz, A., Blasco, J., Fernández-Borràs, J., Planas, J.V., 2013. Effects of sustained swimming on the red and white muscle transcriptome of rainbow trout (Oncorhynchus mykiss) fed a carbohydrate-rich diet. Comparative biochemistry and physiology. Part A, Molecular & integrative physiology, 166, 510–521. https://doi.org/10.1016/j.cbpa.2013.08.005
Mahaliyana, A.S., Fasmina, M.F.A., Alahakoon, A.M.T.B., Wickrama, G.M.G.M., 2016. Toxicity of MSG on embryonic development of zebrafish. International Journal of Scientific Research Publications, 6, 229–234.
Malathi, N., Sooriya, J.J.S., Joseph, C., 2023. The excitotoxic effect of monosodium glutamate on Zebra Fish (Danio rerio). International Journal of Biomolecules and Biomedicine, 17, 7–12.
Miller, L.C., Tainter, M.L., 1944. Estimation of ED50 using logarithmic-probit graph paper. Proceedings of the Society for Experimental Biology and Medicine, 57, 261–264. https://doi.org/10.3181/00379727-57-14776
Monteiro, S.M., Fontainhas-Fernandes, A., Sousa, M., 2010. Immuno-histochemical study of gill epithelial cells in Oreochromis niloticus. Folia Histochemica et Cytobiologica, 48, 112–121. https://doi.org/10.2478/v10042-008-0105-5
Moreno, G., Perelló, M., Gaillard, R.C., Spinedi, E., 2005. Orexin A stimulates HPA axis but not food intake. Endocrine, 26, 99–106. https://doi.org/10.1385/endo:26:2:099
Mukherjee, D., Ferreira, N.G.C., Saha, N.C., 2022. Effects of 2,4,6-trichlorophenol on Clarias batrachus: A biomarker approach. Environmental Science and Pollution Research, 29, 47011–47024. https://doi.org/10.1007/s11356-022-19213-y
Nagelkerken, I., Munday, P.L., 2016. Animal behaviour shapes ecological effects of ocean acidification. Global Change Biology, 22, 974–989. https://doi.org/10.1111/gcb.13167
Niaz, K., Zaplatic, E., Spoor, J., 2018. Extensive use of monosodium glutamate: A threat to public health? EXCLI Journal, 17, 273–278. https://doi.org/10.17179/excli2018-1092
Ninomiya, K., 2002. Umami: a universal taste. Food Reviews International, 18, 23–38. https://doi.org/10.1081/FRI-120003415
Nnadozie, J.O., Chijioke, U.O., Okafor, O.C., Olusina, D.B., Oli, A.N., Nwonu, P.C., Mbagwu, H.O., Chijioke, C.P., 2019. Chronic toxicity of low dose monosodium glutamate in albino Wistar rats. BMC research notes, 12, 593. https://doi.org/10.1186/s13104-019-4611-7
OECD, 2025. Test No. 203: Fish, Acute Toxicity Test, OECD Guidelines for the Testing of Chemicals, Section 2, OECD Publishing, Paris. https://doi.org/10.1787/9789264069961-en
Otomewo, L.O., Adeleke, P.A., Ajayi, A.M., Chimezie, J., Oni, J.O., Eduviere, A.T., Adeoluwa, O.A., Umukoro, S., 2025. Monosodium glutamate aggravates social defeat stress-induced behavioral dysfunctions by promoting neurodegeneration and downregulating prefrontal cortex BDNF expressions in mice. Brain Disorders, 19, 100275. https://doi.org/10.1016/j.dscb.2025.100275
Pandey, S., Kumar, R., Sharma, S., Nagpure, N.S., Srivastava, S.K., Verma, M.S., 2005. Acute toxicity bioassays of mercuric chloride and malathion on air-breathing fish Channa punctatus (Bloch). Ecotoxicology and environmental safety, 61, 114–120. https://doi.org/10.1016/j.ecoenv.2004.08.004
Perumalsamy, N., Nandagopalan, G., Mathan, R., 2024. Histopathological alterations in Labeo rohita exposed to monosodium glutamate (MSG). Journal of Basic and Applied Zoology, 85, 10. https://doi.org/10.1186/s41936-024-00363-z
Porras-Rivera, G., Górski, K., Colin, N., 2024. Behavioural biomarkers in fish for assessing pollution. Environmental Research, 260, 119607. https://doi.org/10.1016/j.envres.2024.119607
Pulido-Reyes, G., Moreno-Martín, G., Gómez-Gómez, B., Navas, J.M., Madrid, Y., Fernández-Cruz, M.L., 2024. Fish acute toxicity of nine nanomaterials: Need of pre-tests to ensure comparability and reuse of data. Environmental Research, 245, 118072. https://doi.org/10.1016/j.envres.2023.118072
Randhawa, M.A., 2009. Calculation of LD50 values from the method of Miller and Tainter, 1944. Journal of Ayub Medical College Abbottabad, 21, 184–185.
Scott, G.R., Sloman, K.A., 2004. The effects of environmental pollutants on complex fish behaviour: integrating behavioural and physiological indicators of toxicity. Aquatic toxicology, 68, 369–392. https://doi.org/10.1016/j.aquatox.2004.03.016
Sharma, M., Rajput, A., Rathod, C., Sahu, S., 2018. Food chemicals induces toxic effect on health: Overview. Pharmaceutical and Biosciences Journal, 6, 33–37. https://doi.org/10.20510/ukjpb/6/i4/177338
Sharma, P., Chadha, P., 2021. Bisphenol A toxicity in Channa punctatus. Saudi Journal of Biological Sciences, 28, 4738–4750. https://doi.org/10.1016/j.sjbs.2021.04.088
Shuhaimi-Othman, M., Yakub, N., Ramle, N.A., Abas, A., 2013. Comparative toxicity of eight metals on freshwater fish. Toxicology and Industrial Health, 31, 773–782. https://doi.org/10.1177/0748233712472519
Singh, S., Rekha, P.D., Arun, A.B., Young, C.C., 2009. Impacts of monosodium glutamate wastewater on plant growth and soil. Ecological Engineering, 35, 1559–1563. https://doi.org/10.1016/j.ecoleng.2009.06.002
Spurgeon, D., Lahive, E., Robinson, A., Short, S., Kille, P., 2020. Species sensitivity to toxic substances: Evolution, ecology and applications. Frontiers in Environmental Science, 8, 588380. https://doi.org/10.3389/fenvs.2020.588380
Stańska, K., Krzeski, A., 2016. The umami taste: from discovery to clinical use. Otolaryngologia Polska, 70. https://doi.org/10.5604/00306657.1199991
Suthamnatpong, N., Ponpornpisit, A., 2017. Effects of monosodium glutamate on heartbeat and zebrafish embryonic development. Thai Journal of Veterinary Medicine, 47, 523–530. https://doi.org/10.56808/2985-1130.2865
United States Environmental Protection Agency, 2016. Ecological Effects Test Guidelines OCSPP 850.1075: Freshwater and Saltwater Fish Acute Toxicity Test. EPA 712-C-16-007.
Vaithilingam, P., Seetharaman, B., Achudhan, A.B., Mudgal, G., Vasantharekha, R., 2025. Chronic exposure to food additives: Monosodium glutamate and tartrazine exposure dysregulates gut–brain axis in zebrafish model. Science of the Total Environment, 998, 180295. https://doi.org/10.1016/j.scitotenv.2025.180295
Vissio, P.G., Darias, M.J., Di Yorio, M.P., Pérez Sirkin, D.I., Delgadin, T.H., 2021. Fish skin pigmentation in aquaculture: The influence of rearing conditions and its neuroendocrine regulation. General and comparative endocrinology, 301, 113662. https://doi.org/10.1016/j.ygcen.2020.113662
Witeska, M., 2024. Neurotoxicity biochemical biomarkers in fish toxicology. Preprints. https://doi.org/10.20944/preprints202409.1610.v1
Wong, B.B.M., Candolin, U., 2015. Behavioural responses to changing environments. Behavioural Ecology, 26, 665–673. https://doi.org/10.1093/beheco/aru183
Yamaguchi, S., Ninomiya, K., 2000. Umami and food palatability. The Journal of Nutrition, 130, 921S–926S. https://doi.org/10.1093/jn/130.4.921S
Zhao, B., Zhao, J., Liu, H., Zhang, H., Shan, H., Zong, J., Cao, Q., Jiang, J., 2025. Impact of Dietary Glutamate on Growth Performance and Flesh Quality of Largemouth Bass. Fishes, 10, 151. https://doi.org/10.3390/fishes10040151
Downloads
Published
Data Availability Statement
The data that supports this research will be shared upon reasonable request to the corresponding authors.
Issue
Section
License
Copyright (c) 2025 Farha Ashique, Pankaj Kumar, Kainat Masih, Amod Kumar, Parimal Kumar Khan

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors retain the copyright to their work and grant the journal and its publisher (Enviro Mind Solutions) a non-exclusive license to publish and distribute the work freely.












